Detail
1. Properties:
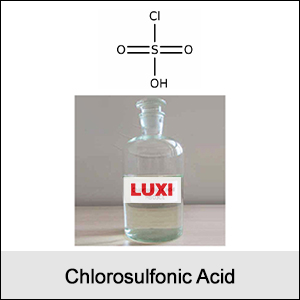
Molecular Formula: ClSO₃H
Molar Mass: 116.52 g/mol
Appearance: Colorless to pale yellow fuming liquid.
Odor: Pungent, irritating odor.
Boiling Point: 151 °C (decomposes at higher temperatures).
Density: 1.75 g/cm³ at 20 °C.
Solubility: Reacts violently with water, releasing hydrogen chloride gas (HCl).
2. Reactivity:
Chlorosulfonic acid is highly reactive due to the presence of both a strong electrophilic sulfonyl chloride group (-SO₂Cl) and a strong acidic hydrogen atom. It is a powerful sulfonating agent and is capable of:
Reacting with organic compounds to introduce a sulfonic acid (-SO₃H) group.
Reacting with alcohols, amines, and phenols to form corresponding sulfonate derivatives.
3. Applications:
Sulfonation Reactions: Used in the production of sulfonic acids, which serve as intermediates in the synthesis of detergents, dyes, and pharmaceuticals.
Chlorinating Agent: Utilized to introduce chlorine into organic molecules.
Manufacture of Surfactants: Key in producing detergent precursors.
Pharmaceuticals: Used in the preparation of certain drugs.
Stabilizers: Acts as a stabilizer in some chemical processes.
4.Hazards and Handling:
Corrosive: Causes severe burns to skin, eyes, and mucous membranes.
Toxic Fumes: Emits hydrogen chloride and sulfur dioxide upon contact with moisture.
Reactive with Water: Can result in violent reactions, releasing toxic and corrosive gases.
Precautions:
Use appropriate personal protective equipment (PPE): gloves, goggles, face shield, and acid-resistant clothing.
Handle in a well-ventilated area or under a fume hood.
Store in tightly sealed containers made of resistant materials, such as Teflon or glass, away from moisture and incompatible materials.